
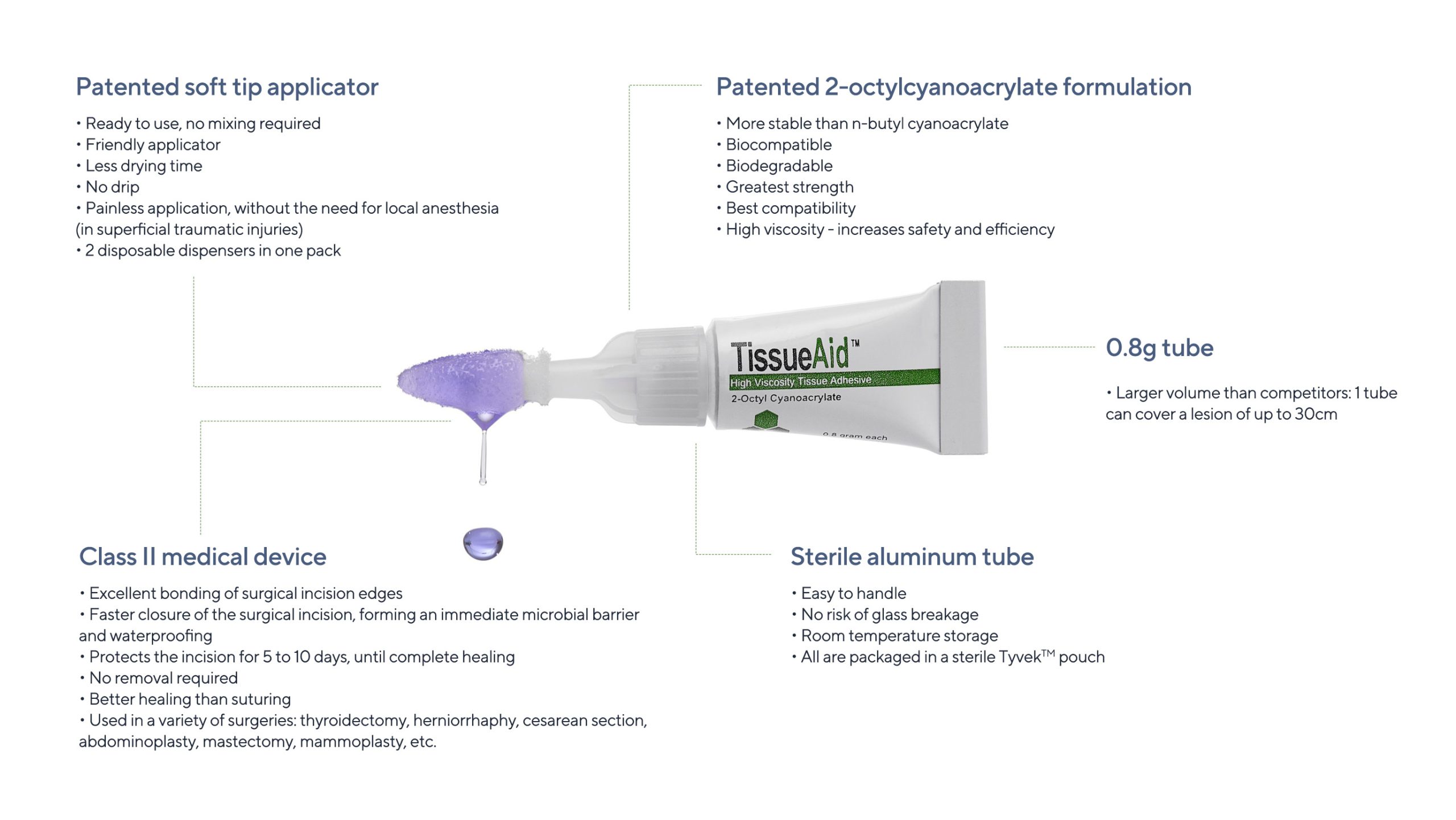
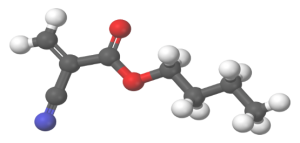
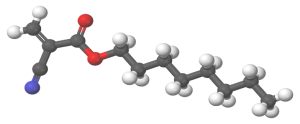
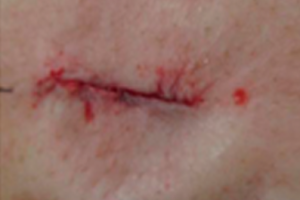
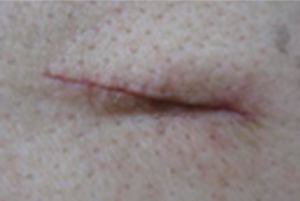
Additionally, 2-octyl cyanoacrylate (2OCA) is approved as an antimicrobial barrier by the US Food & Drug Administration (FDA).




Copyright © Symatese Latam 2023